Uses of Isotopes
Topic Description
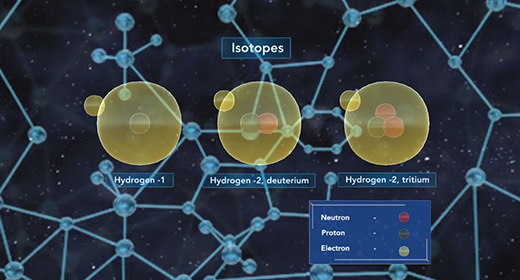
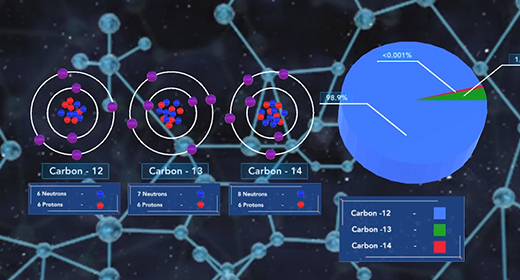
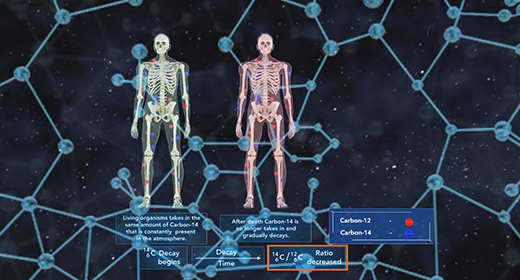
Upon completion of this module, you should be able to know that isotopes differ only by the number of neutrons present in their nuclei and understand how isotopes are used in radioactive dating and isotopic labeling. Isotopes are atoms with the same number of protons but possess a different number of neutrons. Atoms of the element carbon may possess anywhere from 6 to 16 neutrons, but every carbon atom contains six protons. Some isotopes of a particular element are more abundant in nature than others. The abundance of an isotope is related to its stability. Stable isotopes are more abundant in nature because they are less prone to radioactive decay. Carbon-12, with six neutrons, is the most abundant isotope of carbon, followed by carbon-13 and carbon-14.

The topic "Uses of Isotopes" is available as part of this package. Subscribe this package to get access to this topic.