Thermochemistry
Topic Description
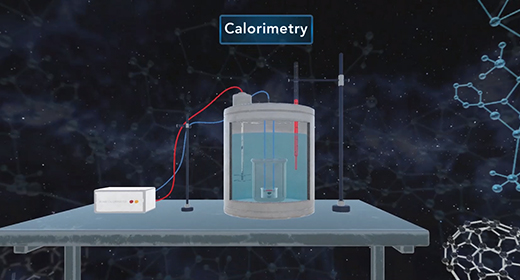
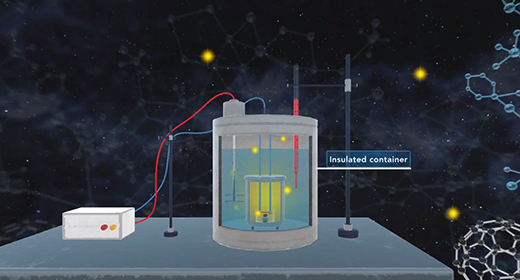
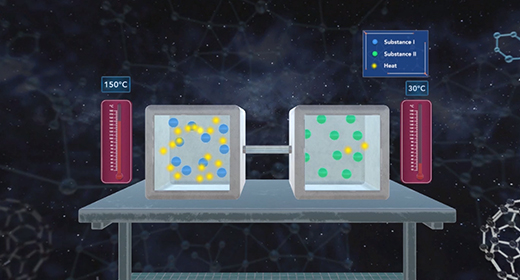
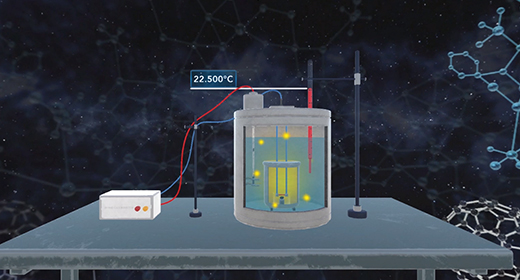
Upon completion of this module, you should be able to understand calorimetry techniques and perform calorimetric calculations. Calorimetry is the part of thermochemistry that deals with measuring the heat involved in chemical changes, physical changes, mixing processes, and transition phases. The word calorimetry is derived from calor, Latin for heat, and [metron], Greek for measurement. Joseph Black a Scottish scientist first made a distinction between temperature and heat, and first established the calorimetry method.

The topic "Thermochemistry" is available as part of this package. Subscribe this package to get access to this topic.