The Second Law of Thermodynamics
Topic Description
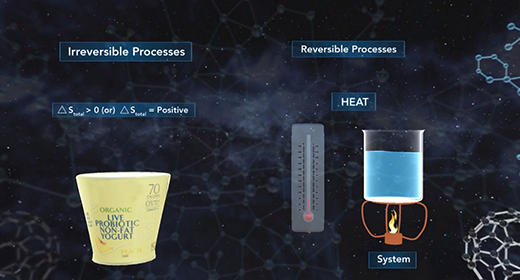
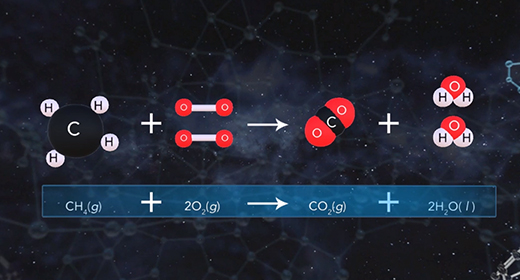
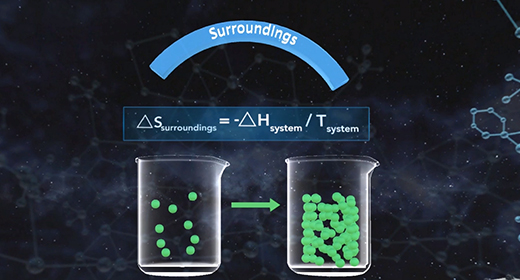
Upon completion of this module, you should be able to understand entropy quantitatively and qualitatively and predict the sign of the system for a given chemical reaction. According to the second law of thermodynamics, no process can have a change in total entropy of less than zero. For irreversible processes, the change in total entropy is greater than zero, while reversible processes have a change in total entropy equal to zero. The change in total entropy is the change in entropy of the universe, which equals the sum of the change in entropy of the system and the change in entropy of the surroundings.

The topic "The Second Law of Thermodynamics" is available as part of this package. Subscribe this package to get access to this topic.