The Rate Law
Topic Description
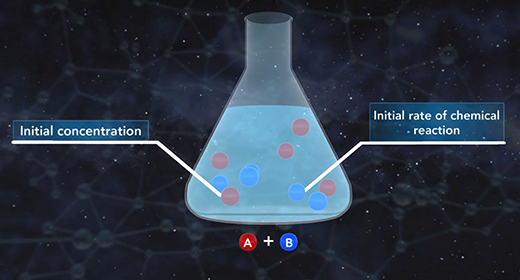
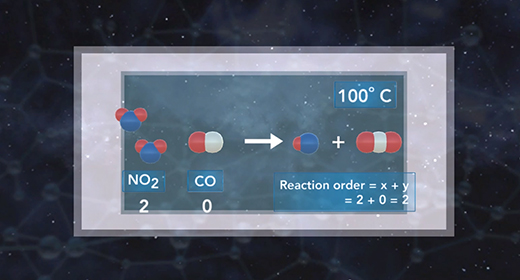
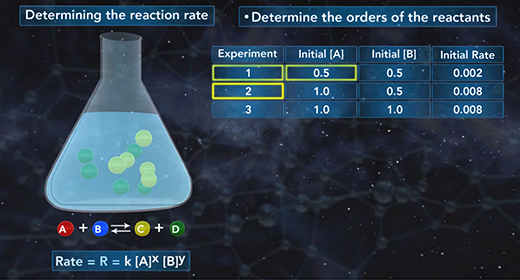
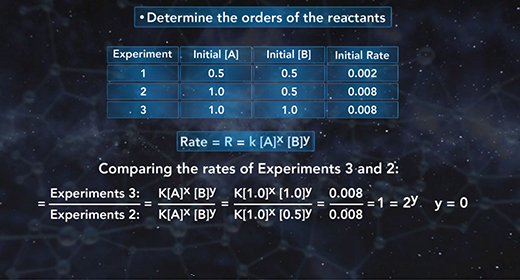
Upon completion of this module, you should be able to write the general expression of the rate law, use the method of initial rates to calculate the rate constant, k, of a chemical reaction, and the order of each reactant and determine the overall order of a reaction. The rate law shows the relationship between the initial rate of a chemical reaction and the initial concentrations of the reactants. The general expression of the rate law is shown here. The initial rate of a chemical reaction is equal to the rate constant, k, times the initial concentrations of the reactants raised to a power.

The topic "The Rate Law" is available as part of this package. Subscribe this package to get access to this topic.