The Pressure of a Gas
Topic Description
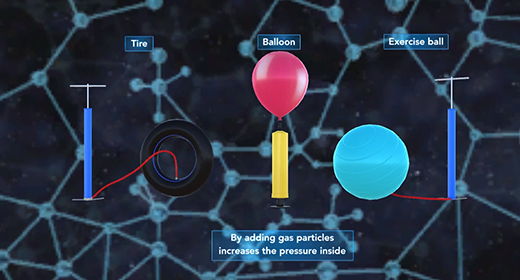
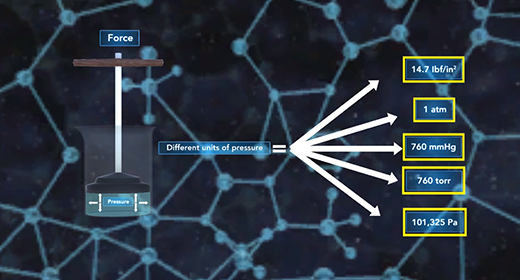
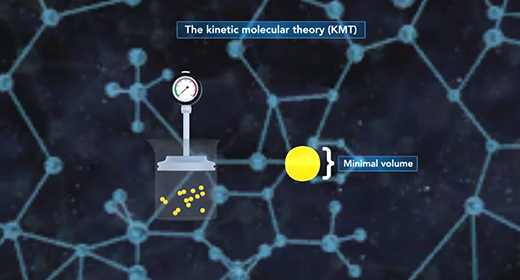
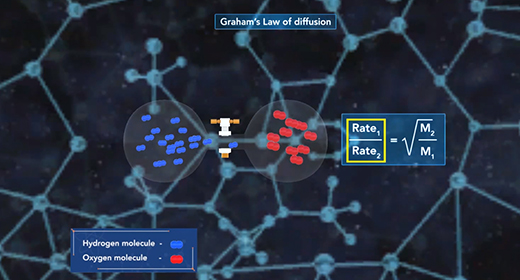
Upon completion of this module, you should be able to explain what causes pressure and interconvert between units of pressure, understand how a barometer works, and Describe the Kinetic Molecular Theory and relate it to Graham’s Law. Pressure (P) is the force of gas particles per unit area. Tires, balloons, and exercise balls increase in volume as you increase the number of gas particles in them. This increases the pressure inside; and this increased pressure inflates them. Pressure can be expressed using different units of pressure. These units include atmospheres (atm), millimeters of mercury (mmHg), torr, pounds per square inch (lb/in2), and Pascals (Pa)

The topic "The Pressure of a Gas" is available as part of this package. Subscribe this package to get access to this topic.