The Ideal Gas Equation
Topic Description
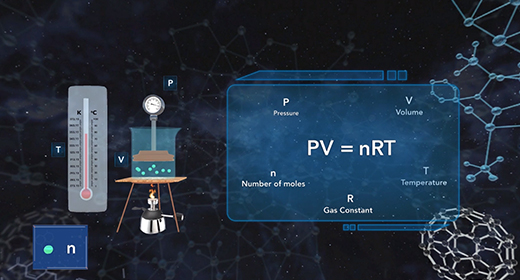
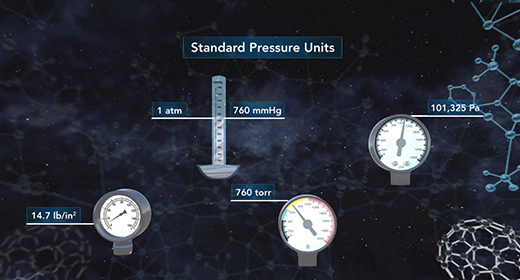
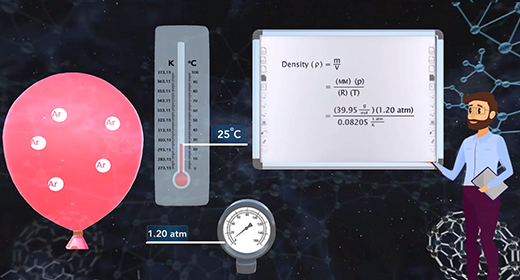
Upon completion of this module, you should be able to understand the ideal gas law equation and choose the correct form of the gas constant. Émile Clapeyron (1799–1864) was able to combine Avogadro's Law, together with three other gas laws including Boyle's Law, Charles's Law, and Gay-Lussac's Law to introduce the ideal gas law. The modern form of the ideal gas law is PV is equal to nRT where P is the pressure of a gas, V is the volume, n is the number of moles of gas, R is the universal gas constant, and T is the absolute temperature.

The topic "The Ideal Gas Equation" is available as part of this package. Subscribe this package to get access to this topic.