The Effect of Temperature on Solubility
Topic Description
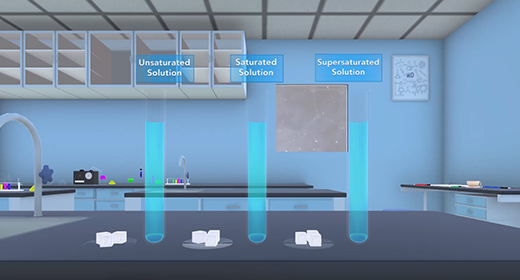
Upon completion of this module, you should be able to define solubility, understand some factors that affect solubility, and understand the effect of temperature on solubility. Solubility is the maximum amount of solute, such as sodium chloride, that can dissolve in a specific volume of a solvent, such as water, at a given temperature. Solutions could be unsaturated, saturated, or supersaturated. Unsaturated solutions have less solute than the maximum amount dissolved at a specific temperature. Saturated solutions have the maximum soluble amount of solute at a specific temperature. Supersaturated solutions have more soluble solute than is usually possible at that temperature.

The topic "The Effect of Temperature on Solubility" is available as part of this package. Subscribe this package to get access to this topic.