Standard Reduction Potentials
Topic Description
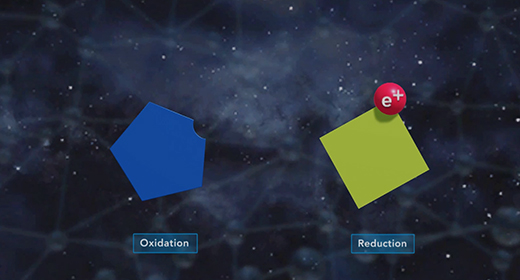
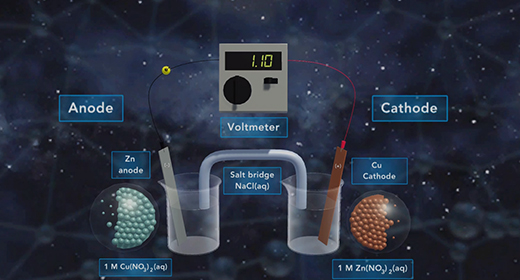
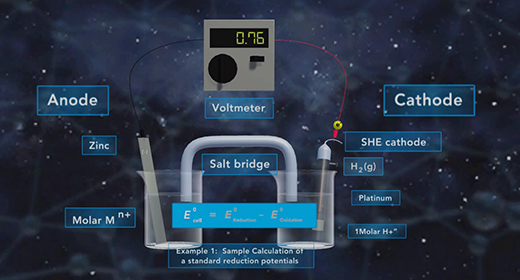
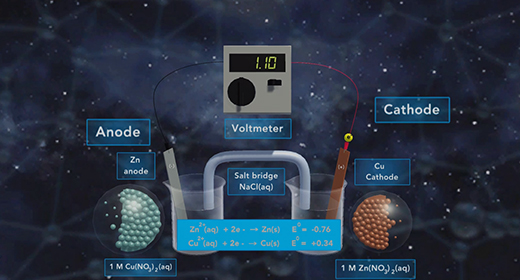
Upon completion of this module, you should be able to determine the direction of electron flow in a galvanic cell, define standard reduction potential and calculate standard reduction potentials Oxidation is the Loss of Electrons, and Reduction is the Gain of Electrons. The anode is the electrode where oxidation happens. The cathode is the electrode where reduction happens. The flow of electrons in a galvanic cell is always from Anode to Cathode, and the process is spontaneous. The flow of electrons in a galvanic cell is always from the anode to the cathode because the cathode has a lower electrical potential energy than the anode.

The topic "Standard Reduction Potentials" is available as part of this package. Subscribe this package to get access to this topic.