Solubility Equilibria
Topic Description
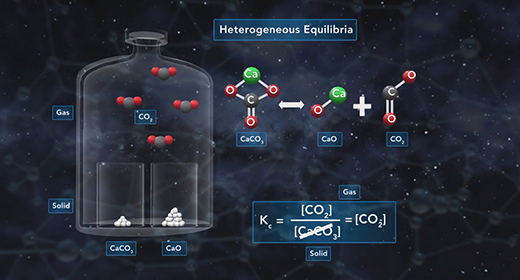
Upon completion of this module, you should be able to explain the difference between solubility and molar solubility, write equilibrium expressions for solubility equilibria, distinguish between Ksp and Q, and solve problems involving the solubility product, Ksp. Molar solubility is the number of moles of solute dissolved in one liter of a saturated solution. Solubility is the number of grams of solute dissolved in one liter of a saturated solution.

The topic "Solubility Equilibria" is available as part of this package. Subscribe this package to get access to this topic.