Radial Probability Function
Topic Description
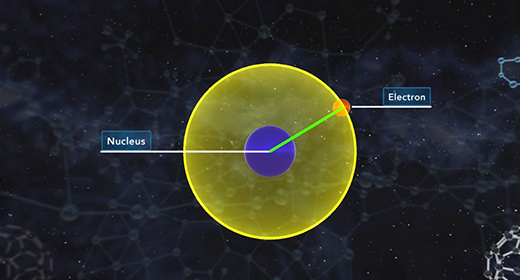
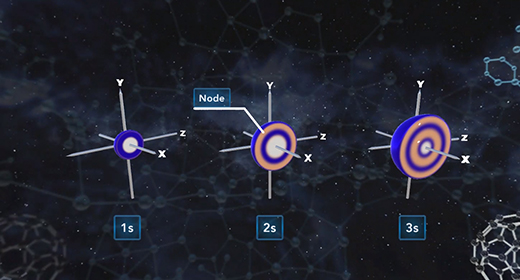
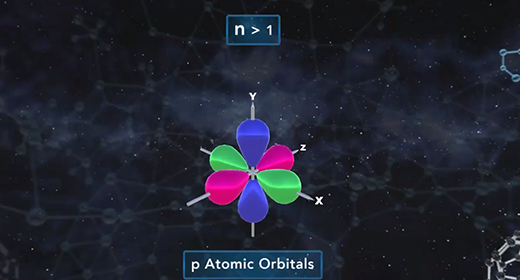
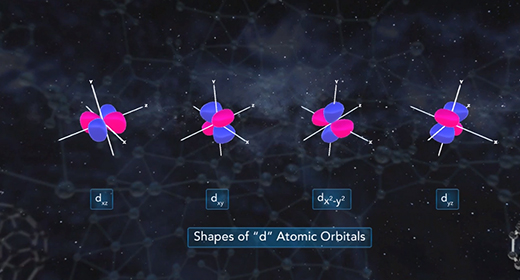
Upon completion of this module, you should be able to know how quantum mechanics determines the probable positions of electrons within the orbitals of an atom. R is the distance between the nucleus and electron and is typically measured in angstroms. All orbitals, except the 1s orbital, possess nodes. A node is a region or point in the orbital where the probability that an electron is located equals zero. For s orbitals, the number of nodes present is equal to n-1.

The topic "Radial Probability Function" is available as part of this package. Subscribe this package to get access to this topic.