Quantum Number
Topic Description
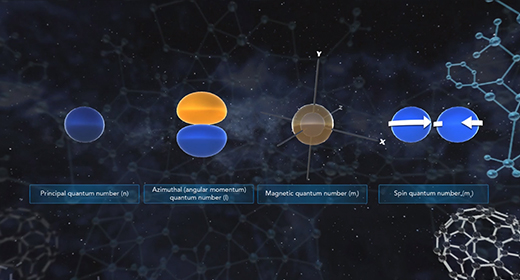
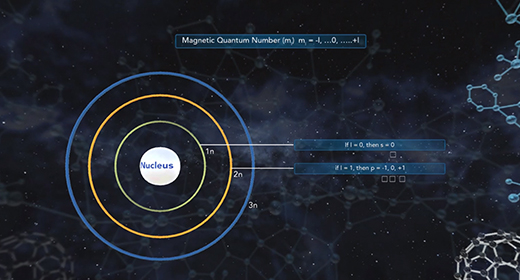
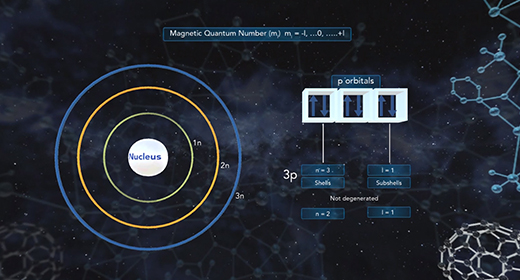
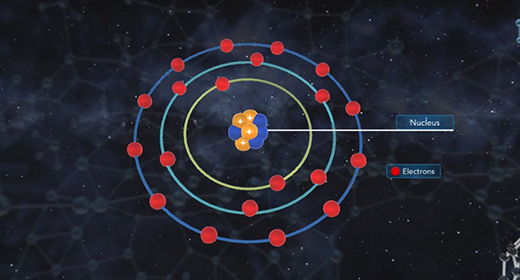
Upon completion of this module, you should be able to know the different kinds of quantum numbers and what each represents and differentiate between shell, types of orbitals, orientation, and electron spin. Quantum numbers describe the probable locations of electrons in an atom. Each electron in an atom can be assigned four quantum numbers: a principal quantum number (n), an azimuthal (angular momentum) quantum number (l), a magnetic quantum number (ml), and a spin quantum number (ms). Every electron in an atom will have a unique set of quantum numbers (n, l, ml, ms); and no two electrons in the same atom will have the same four quantum numbers (Pauli Exclusion Principle).

The topic "Quantum Number" is available as part of this package. Subscribe this package to get access to this topic.