Precipitation Reactions
Topic Description
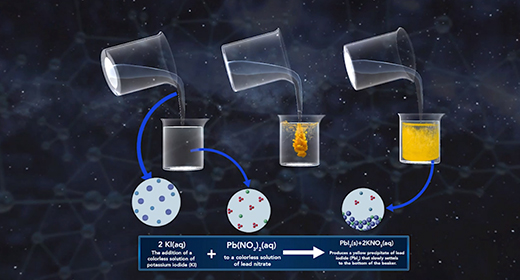
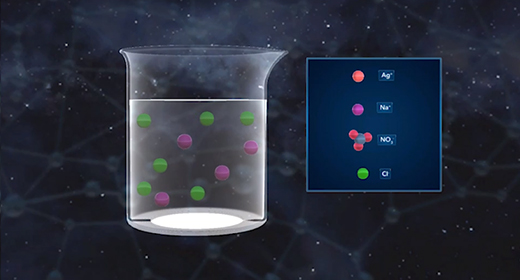
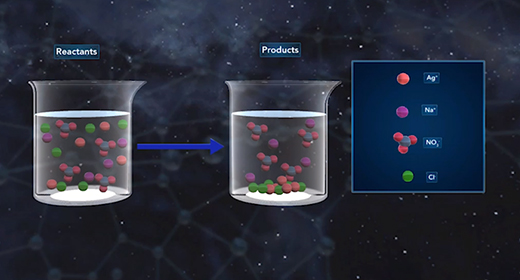
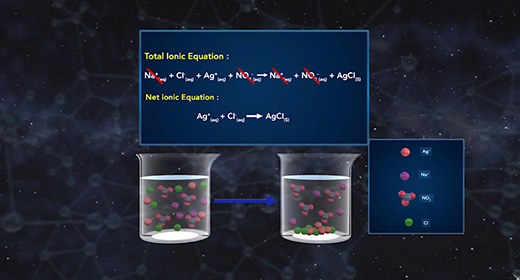
Upon completion of this module, you should be able to recognize the chemical composition of a precipitate, understand how precipitates form and how solubility affects the formation of precipitates, and understand how to write ionic and net ionic equations. In a precipitation reaction, ionic particles in solution react to form a precipitate. A precipitate is a solid substance produced from reactions in solution. The reactants in precipitation reactions are dissolved ions. If the attraction between the negatively and positively charged ions is strong enough, they form ionic bonds, yielding an ionic compound. The resulting solid compound comes out of the solution as a precipitate.

The topic "Precipitation Reactions" is available as part of this package. Subscribe this package to get access to this topic.