Nomenclature of Ionic compounds
Topic Description
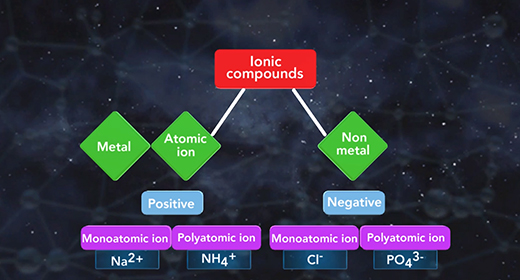
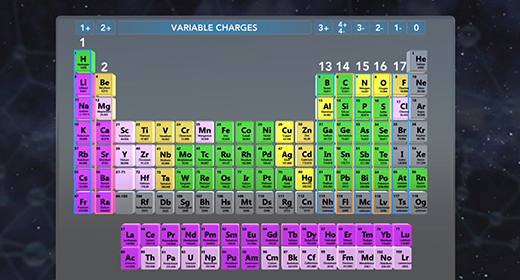
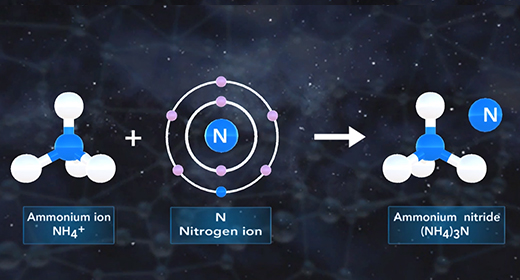
Upon completion of this module, you should be able to name a compound, given the formula, and write the formula, given the name. An ionic compound is composed of two ions. A positive ion is a cation, and a negative ion is an anion. An ionic compound must contain metal or a positive polyatomic ion such as ammonium (NH4+) and a nonmetal ion or a negative polyatomic ion such as phosphate PO4 3-. The ionic formula is the lowest whole number ratio in which these atoms combine.

The topic "Nomenclature of Ionic compounds" is available as part of this package. Subscribe this package to get access to this topic.