Nature of Nuclear Reactions
Topic Description
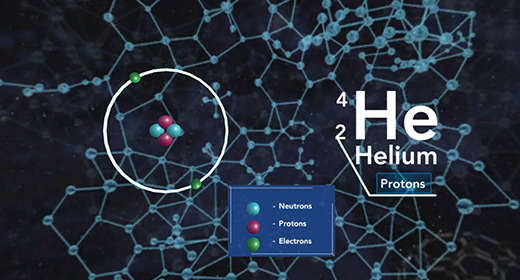
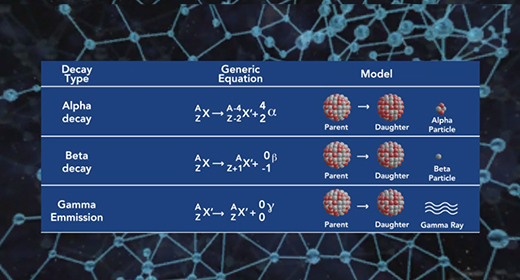
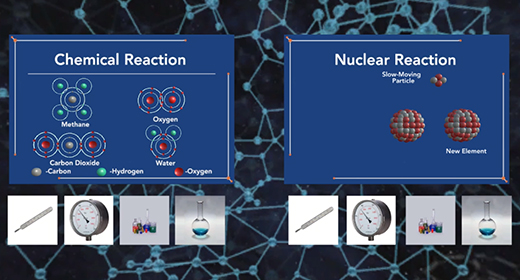
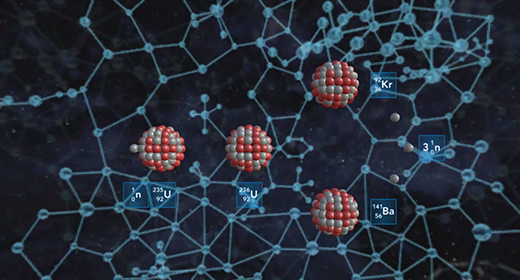
Upon completion of this module, you should be able to compare and contract chemical reactions and nuclear reactions and balance nuclear reactions. The atomic number (Z) of an atom represents the number of protons in the nucleus. The mass number (A) of an element is the number of protons and the number of neutrons (atomic number Z + the number of neutrons, N). Nuclear or radioactive decay happens when an unstable atom loses energy by emitting ionizing radiation, including alpha, beta, and gamma decays.

The topic "Nature of Nuclear Reactions" is available as part of this package. Subscribe this package to get access to this topic.