Molecular Structure and the Strength of Acids
Topic Description
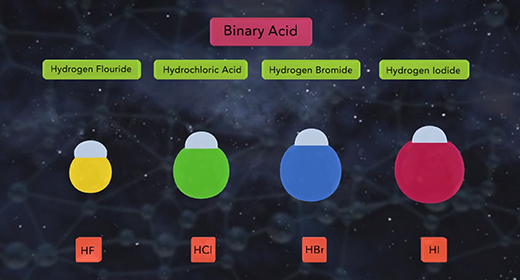
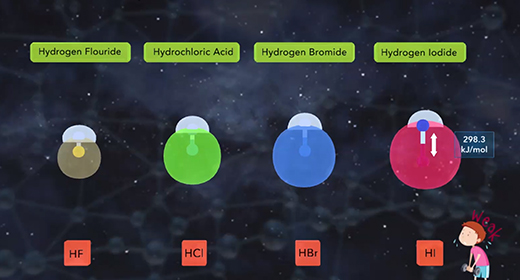
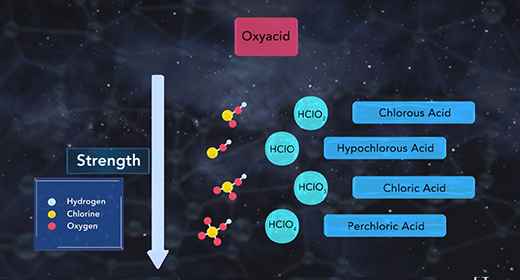
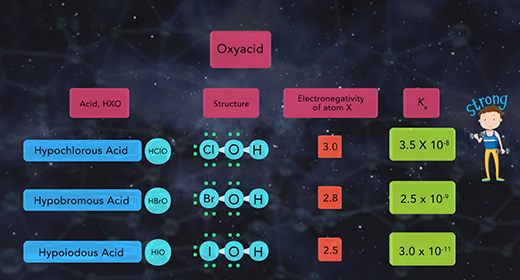
Upon completion of this module, you should be able to describe the factors affecting the acid strength of binary acids and describe the factors affecting the acidity of the oxoacids. Binary acids are acids with hydrogen combined with a non-metallic element. Binary acids include hydrogen fluoride, HF, a weak acid, as well as hydrochloric acid, HCl, hydrogen bromide, HBr, and hydrogen iodide, HI, all strong acids. Two factors affect binary acid strength. The first factor is the bond polarity. The higher the bond polarity, the higher the acid strength. The second factor is bond strength. The lower the bond strength, the higher the acidity. The chart shown compares the binary acids of group 7A. The chart shows that the bond strength increases as the size of the halogen atom decrease.

The topic "Molecular Structure and the Strength of Acids" is available as part of this package. Subscribe this package to get access to this topic.