Molecular Geometry
Topic Description
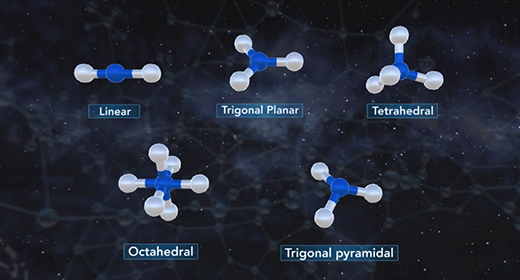
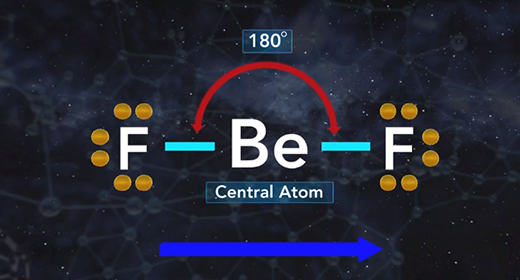
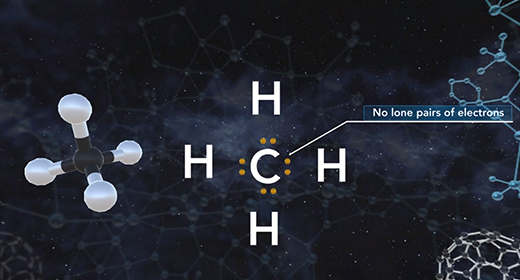
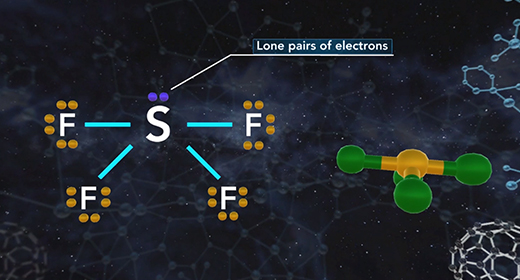
Upon completion of this module, you should be able to determine the electron domain geometry of a molecule or ion from the Lewis structure, determine the shape of a molecule or ion using shared and lone pairs of electrons and determine the bond angles in a molecule or ion. Molecular geometry is the three-dimensional shape of a molecule and is crucial because it determines important characteristics of a molecule such as physical and chemical properties. The Valence Shell Electron Pair Repulsion (VSEPR) Theory allows the molecular shape to be predicted using Lewis structures. The shape is determined using these rules. The Lewis structure of a molecule or ion is determined. Count the number of shared pairs of electrons and lone pairs of electrons.

The topic "Molecular Geometry" is available as part of this package. Subscribe this package to get access to this topic.