Ionization Constants and Conjugate Bases
Topic Description
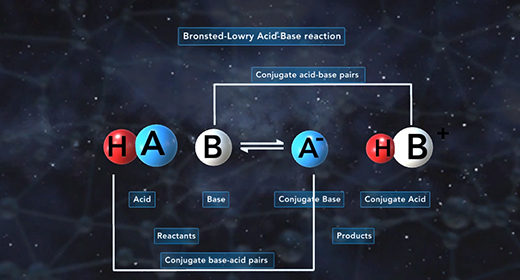
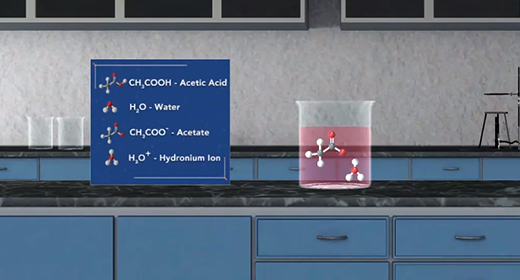
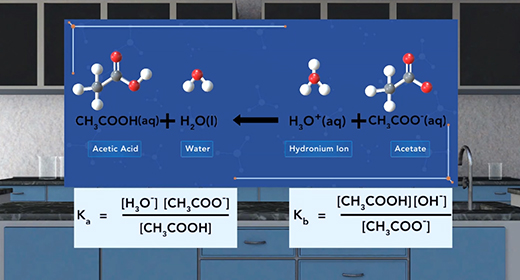
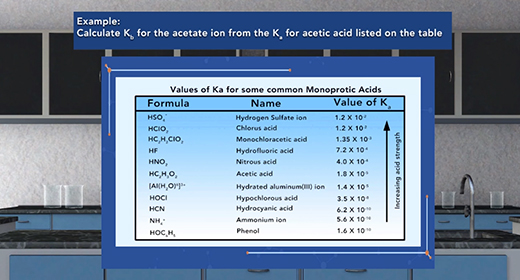
Upon completion of this module, you should be able to identify conjugate acid-base pairs and understand the relationship between the ionization constants of conjugate acid-base pairs. Bronsted-Lowry defined an acid as a proton (H+ ion) donor and a base as a proton acceptor. For example, acetic acid, CH3COOH, acts as an acid (it donates a proton or H+ ion) when placed in water, H2O, which accepts H+ ion and acts as Bronsted-Lowry base. This reaction produces acetate ions (CH3COO-) and hydronium ions (H3O+). For the reverse reaction, the acetate ion (CH3COO-) serves as a base because it accepts a proton from the hydronium ion, H3O+, to form acetic acid (CH3COOH) and water.

The topic "Ionization Constants and Conjugate Bases" is available as part of this package. Subscribe this package to get access to this topic.