Hybridization of Atomic Orbitals
Topic Description
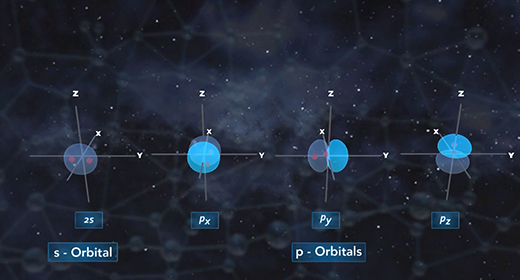
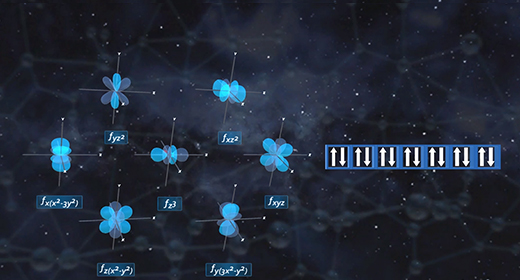
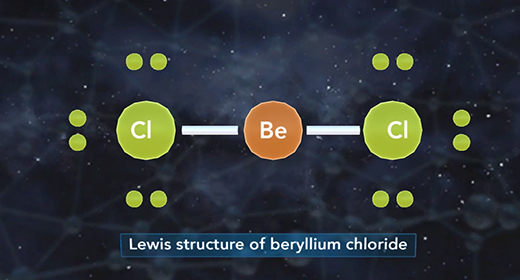
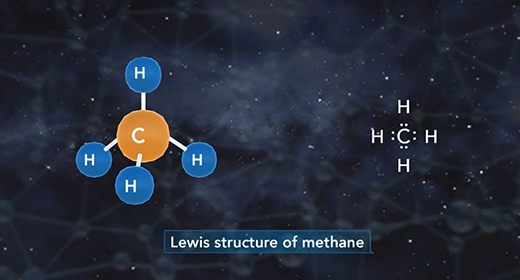
Upon completion of this module, you should be able to define hybridization and predict if an atom has sp, sp2, or sp3 hybridization. Hybridization is the mixing of two or more atomic orbitals to form a new set of hybrid orbitals. When two or more atomic orbitals combine, a hybrid orbital with different shape forms. The Shape of Atomic Orbitals: The s orbitals of an atom are spherical in shape and can hold a maximum of two electrons. The orbital with the lowest energy is the 1s orbital. If these orbital holds one electron, the electron configuration is written 1s1; if it contains two electrons, it is written 1s2.

The topic "Hybridization of Atomic Orbitals" is available as part of this package. Subscribe this package to get access to this topic.