Heat of Dilution
Topic Description
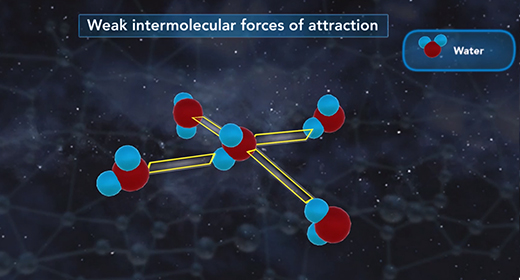
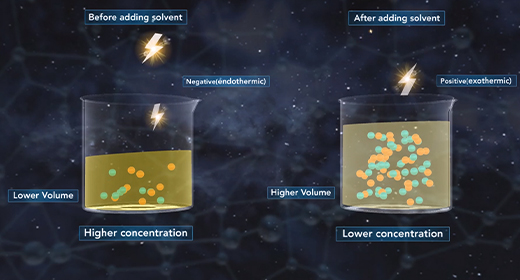
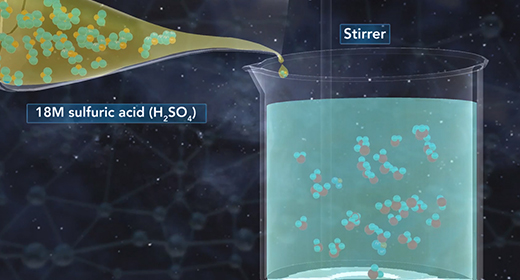
Upon completion of this module, you should be able to understand the heat of dilution and know the difference between endothermic and exothermic reactions. Solid sodium chloride forms a crystal lattice in which sodium ions and chloride ions are attracted to each other by ionic bonds. This is a very ordered, three-dimensional arrangement of ions. The dissolution of solid sodium chloride in water involves three steps.

The topic "Heat of Dilution" is available as part of this package. Subscribe this package to get access to this topic.