Gibbs Free Energy and Spontaneity
Topic Description
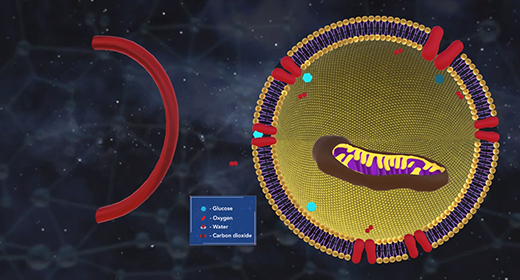
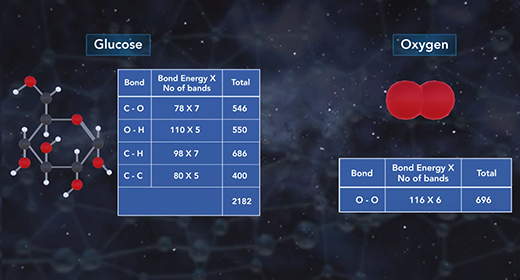
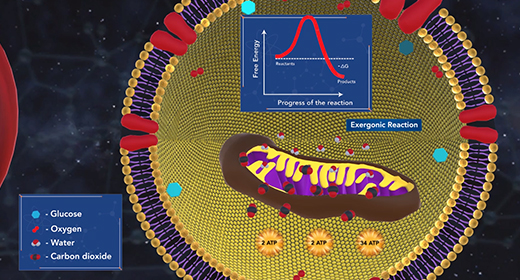
Upon completion of this module, you should understand the relationship between Gibbs free energy and spontaneity. Entropy alone cannot determine the spontaneity of a thermodynamic process. Gibbs free energy, G, quantifies the work that can be performed by a system at constant pressure in temperature. The change in G, delta G, for a process can be used to definitively determine whether the process is spontaneous. A negative delta G signifies a spontaneous process, while a positive delta G indicates a nonspontaneous process.

The topic "Gibbs Free Energy and Spontaneity" is available as part of this package. Subscribe this package to get access to this topic.