Gas Stoichiometry
Topic Description
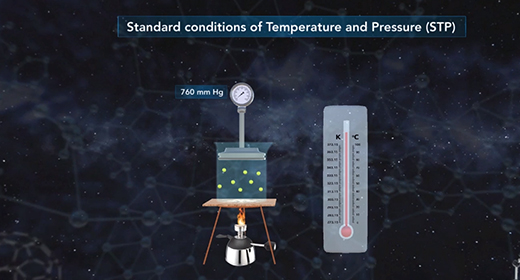
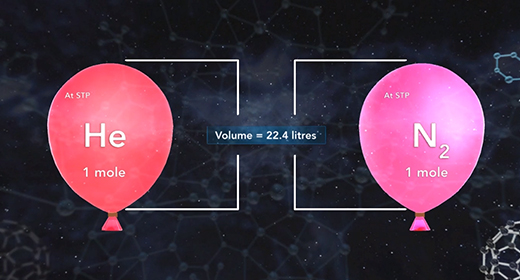
Upon completion of this module, you should be able to solve problems using standard temperature-pressure conditions and solve problems using standard molar volume. The ideal gas law shows the relationship between all the properties of a gas. PV is equal to nRT where P is the pressure, V is the volume, n is the number of moles of gas, T is the temperature of the gas expressed in Kelvins, and R is the universal gas constant.

The topic "Gas Stoichiometry" is available as part of this package. Subscribe this package to get access to this topic.