Formal Charge and Lewis Structure
Topic Description
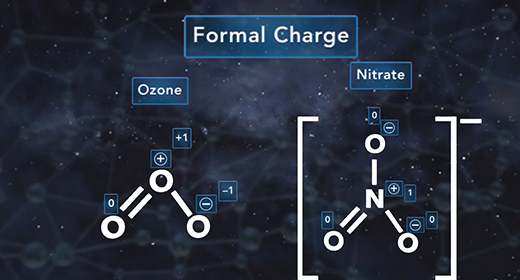
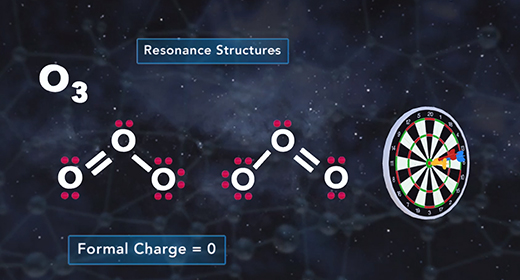
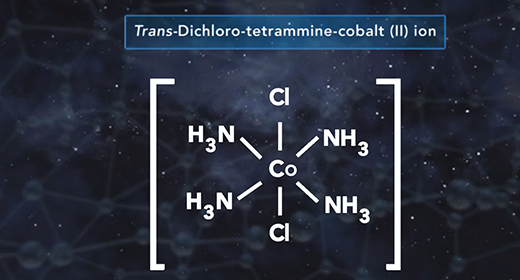
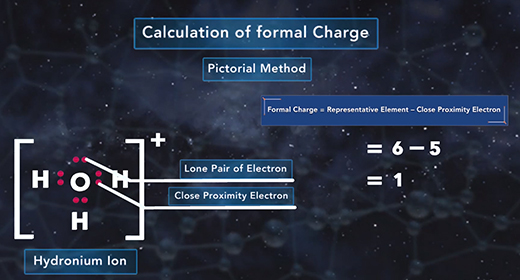
Upon completion of this module, you should be able to determine the formal charge of a given atom in a Lewis structure. The formal charge of an element is the electric charge of that element in a compound. It is used to determine the electronic distribution for the entire molecule or ion. The formal charge is mainly used when there are resonance structures or when different Lewis structures exist. The closer the formal charge is to zero, the lower the energy of the element, and the more accurate is the Lewis structure for the molecule or ion. Relative electronegativity or octet rule is not considered when expressing a formal charge.

The topic "Formal Charge and Lewis Structure" is available as part of this package. Subscribe this package to get access to this topic.