Entropy
Topic Description
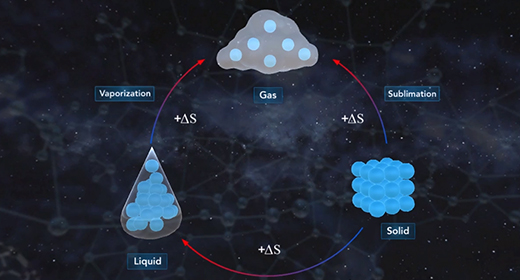
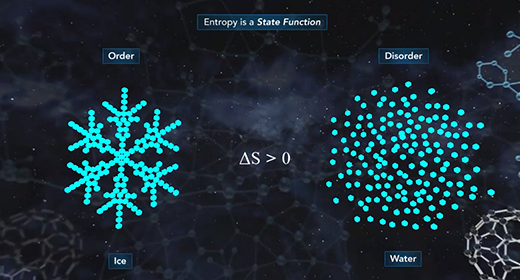
Upon completion of this module, you should be able to define entropy, explain what is meant by a state function, and describe the first, second, and third laws of thermodynamics. Entropy (S) is a measure of the magnitude of the randomness or disorder of a system. If a chemical or a physical change results in an increase in randomness, the entropy change, delta S (?S), is positive. If there is a decrease in randomness, the entropy change, delta S (?S), is negative. The solid-state of any substance is more ordered than the liquid state. The liquid state of any material is more ordered than the gas state.

The topic "Entropy" is available as part of this package. Subscribe this package to get access to this topic.