Electronegativity
Topic Description
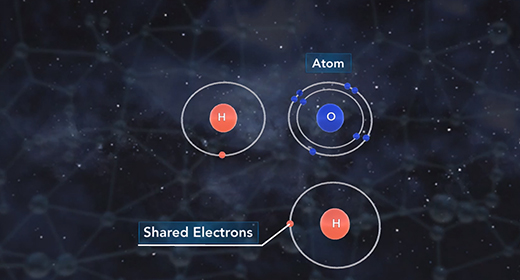
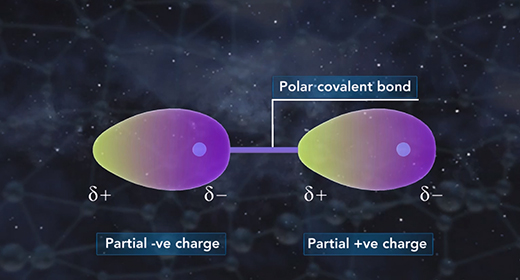
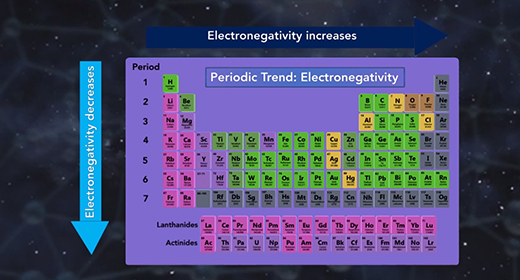
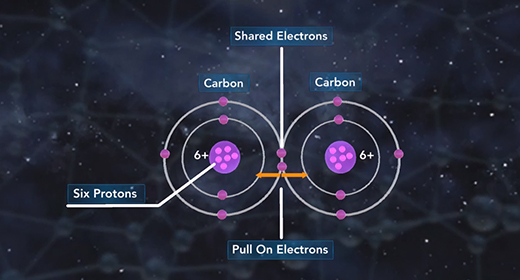
Upon completion of this module, you should be able to differentiate between different types of chemical bonds based on electronegativity and understand the trend of electronegativity in the Periodic Table. Electronegativity is the measure of how well an atom attracts shared electrons in a bond. When atoms of similar electronegativity react, they tend to produce covalent bonds in which electrons are shared between atoms.

The topic "Electronegativity" is available as part of this package. Subscribe this package to get access to this topic.