Electrolysis
Topic Description
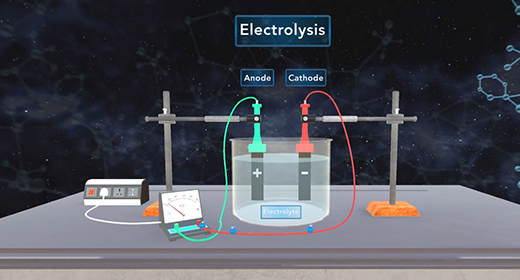
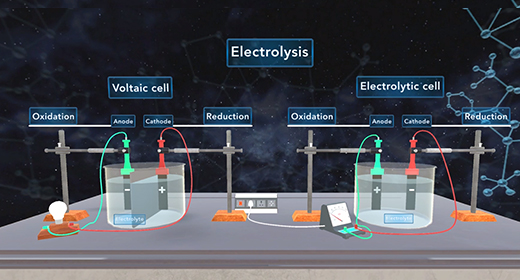
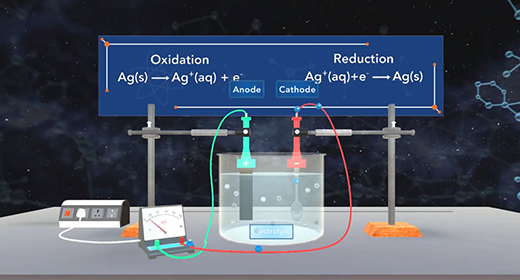
Upon completion of this module, you should be able to define electrolysis, explain the difference between voltaic and electrolytic cells, explain the process of electroplating, and solve problems involving electroplating. Electrolysis is a process that utilizes an external current to force a nonspontaneous reaction to proceed. What is the difference between voltaic cells and electrolytic cells? In both voltaic and electrolytic cells, the reduction occurs at the cathode; and oxidation occurs at the anode.

The topic "Electrolysis" is available as part of this package. Subscribe this package to get access to this topic.