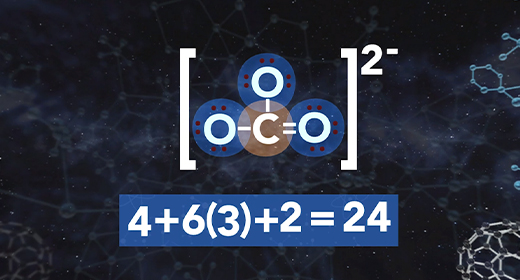Drawing Lewis Structures
Topic Description
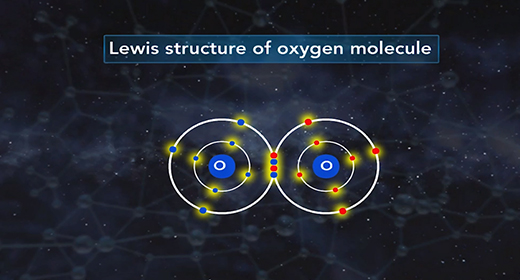
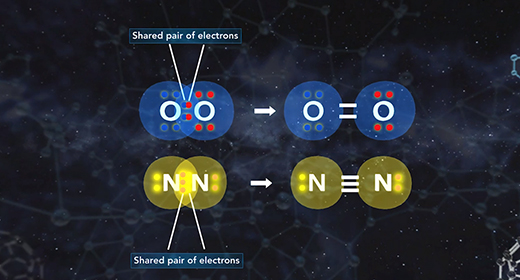
On completion of this module, you should be able to draw the Lewis structure of a given molecule. A Lewis structure is a drawing showing the covalent bonds resulting from valence electrons. A dash represents a shared pair of electrons. One shared pair of electrons forms a single bond represented by a single dash. A double dash represents a double bond, and a triple dash represents a triple covalent bond. A pair of dots shows a lone pair of valence electrons. A covalent bond forms between two non-metals or between a metalloid and a non-metal.

The topic "Drawing Lewis Structures" is available as part of this package. Subscribe this package to get access to this topic.