Corrosion
Topic Description
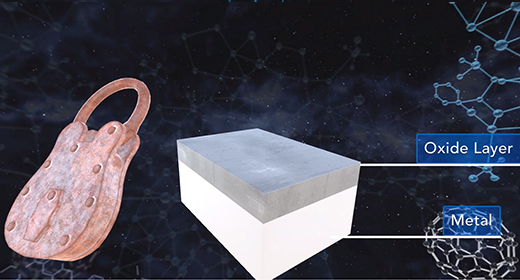
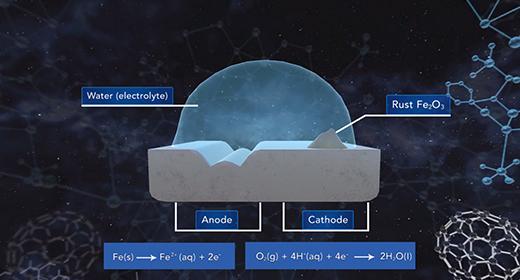
Upon completion of this module, you should be able to explain what occurs when metals corrode, describe the chemical reactions that occur when iron corrodes, and describe ways to prevent corrosion. Corrosion is the spontaneous oxidation of the metal and usually involves oxygen. Many metals develop a thin layer of oxide on their surfaces that protect the inner atoms of these metals. Some metals, including copper, silver, gold, and platinum are resistant to oxidation.

The topic "Corrosion" is available as part of this package. Subscribe this package to get access to this topic.