Chemical Reactions and Chemical Equation
Topic Description
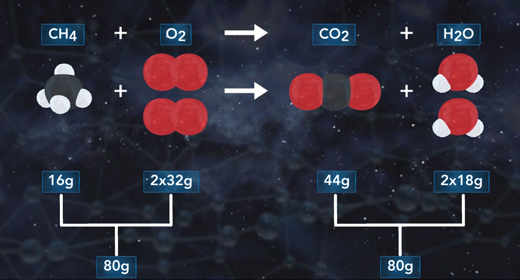
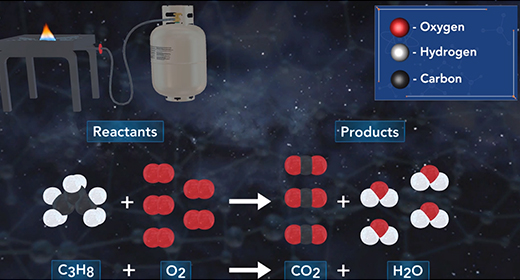
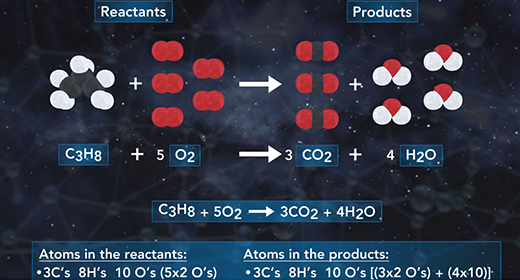
Upon completion of this module, you should be able to write and balance chemical equations. A chemical reaction is a process in which one or more substances collide to produce one or more new substances. In a chemical equation, the reactants are on the left side and the products on the right. Reactants and products are separated by an arrow. Coefficients are whole numbers that show how many atoms or molecules of a given element or compound react and are formed. The law of conservation of mass states that atoms cannot be created or destroyed in a chemical reaction; therefore, the number of atoms of each element must be the same on both sides of the chemical equation. A chemical reaction can be written using a chemical equation that contains chemical symbols to illustrate what happens during a chemical reaction.

The topic "Chemical Reactions and Chemical Equation" is available as part of this package. Subscribe this package to get access to this topic.