Building Up Principle
Topic Description
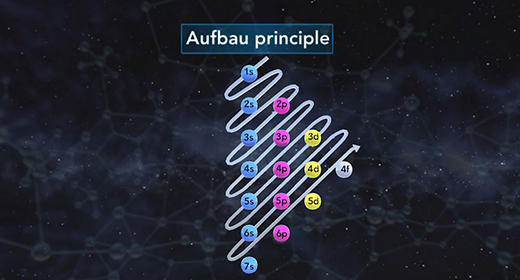
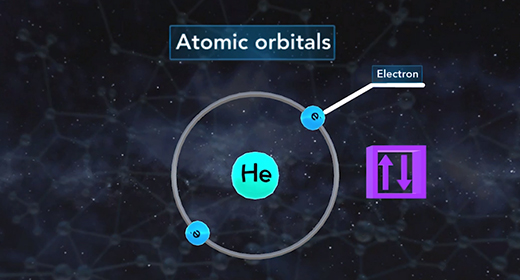
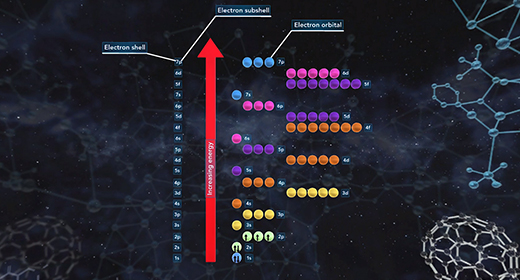
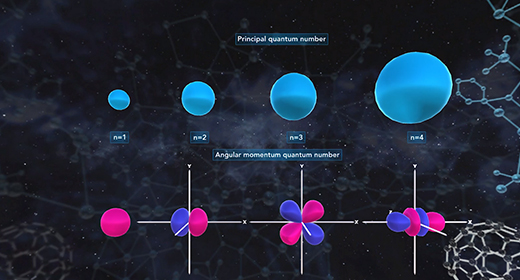
Upon completion of this module, you should be able to understand that atomic orbitals are filled with electrons in order of increasing energy and understanding of the principal quantum number and angular momentum number. The building-up principle also called the Aufbau principle, asserts that the orbitals of an atom in its ground state are filled with electrons in order of increasing energy. Atomic orbitals are defined regions outside of the nucleus that may be occupied by a maximum of two electrons. According to the building-up principle, an orbital with given energy must be filled with two electrons before the orbital with the next highest energy is filled.

The topic "Building Up Principle" is available as part of this package. Subscribe this package to get access to this topic.