Bronsted Lowery Acids and Bases
Topic Description
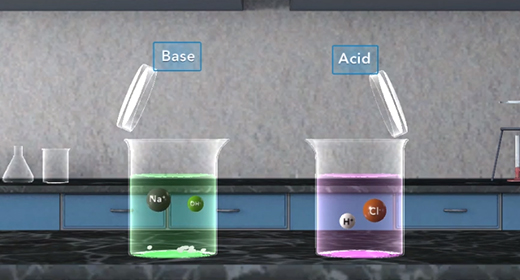
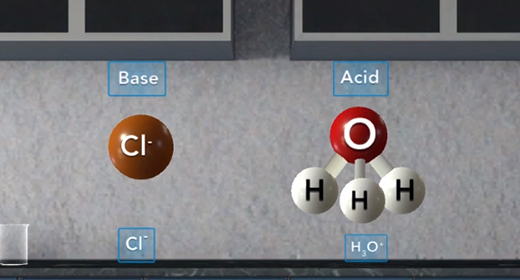
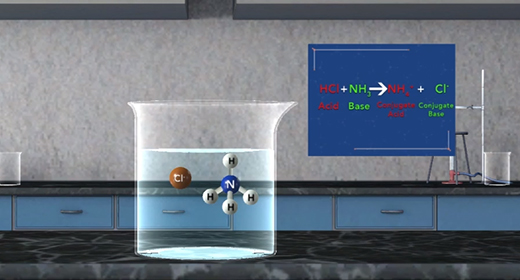
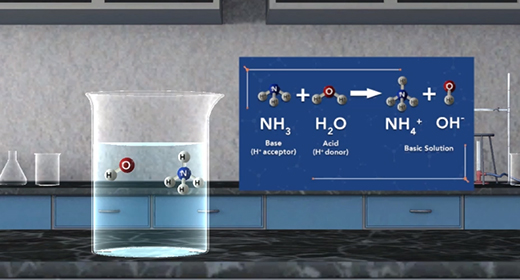
Upon completion of this module, you should be able to define and identify Arrhenius acids and bases and identify Bronsted-Lowry acids and bases. Svante Arrhenius, a Swedish chemist (1859-1927), defined an acid as a substance that produces hydrogen ions in solution and a base as a substance that produces hydroxide ions in solution. Thus, as shown in these reactions, HCl is an acid, and NaOH is a base, HCl produces H plus and Cl minus. NaOH produces Na plus and OH minus.

The topic "Bronsted Lowery Acids and Bases" is available as part of this package. Subscribe this package to get access to this topic.