Batteries and Fuel Cells
Topic Description
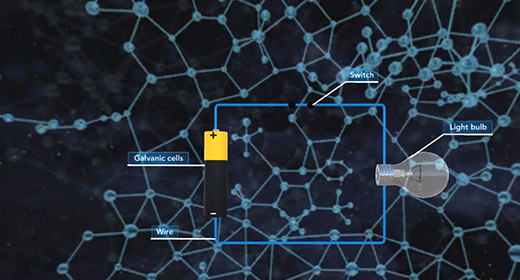
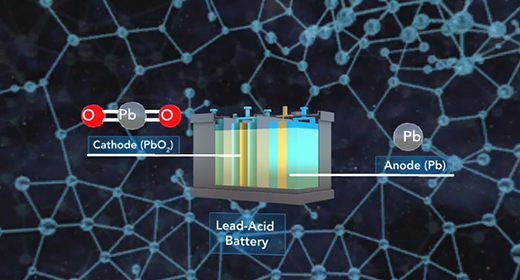
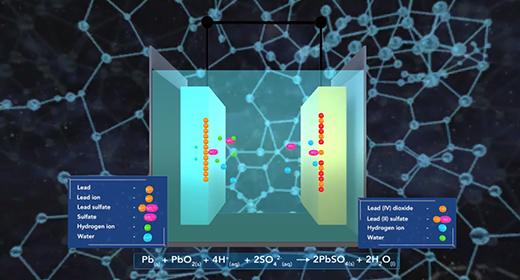
Upon completion of this module, you should be able to identify the components of different types of batteries and fuel cells and explain how batteries and fuel cells work. A battery is a self-contained electrochemical power source with one or more voltaic cells, also called galvanic cells. The flow of electrons in a galvanic cell is always from “Anode to Cathode,” and the process is spontaneous. The electrons flow from anode to cathode because the cathode has a lower electrical potential energy than the anode. The electromotive force (emf) is the force required to move electrons through the external circuit. When cells are connected in series, greater E M Fs are achieved.

The topic "Batteries and Fuel Cells" is available as part of this package. Subscribe this package to get access to this topic.