Amounts of Reactants-Products
Topic Description
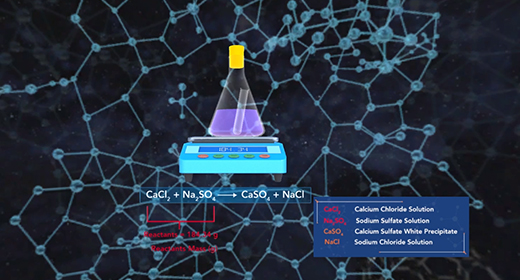
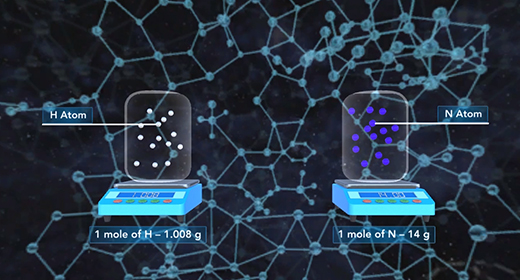
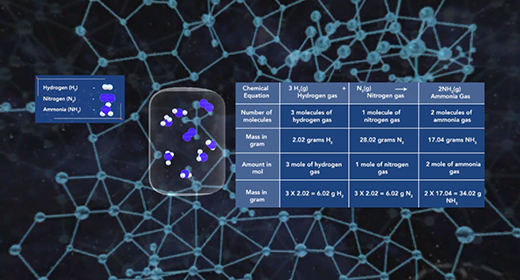
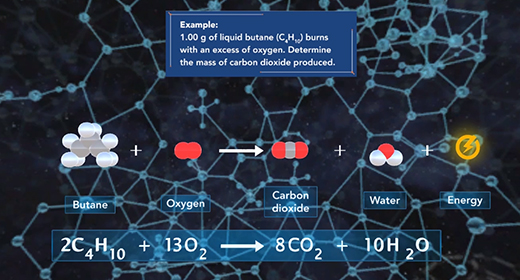
Upon completion of this module, you should be able to determine the amounts of reactants and products involved in a reaction. The law of conservation of mass states that the mass of a system remains the same if the system is closed. The mass of the reactants before the chemical reaction must equal the mass of the products after the reaction. Since a chemical reaction occurs when new substances form, no new atom is created, and no former element can disappear; some bonds break, and new ones form, but the total mass remains the same. The original atoms rearrange themselves to form new substances.

The topic "Amounts of Reactants-Products" is available as part of this package. Subscribe this package to get access to this topic.