Amorphous Solid
Topic Description
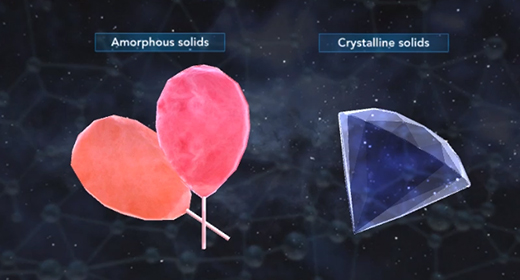
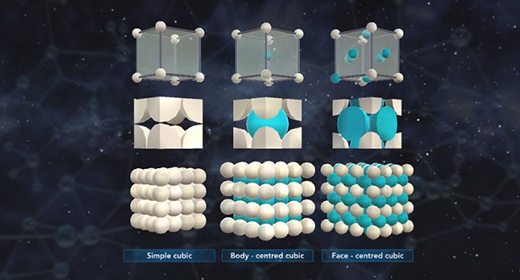
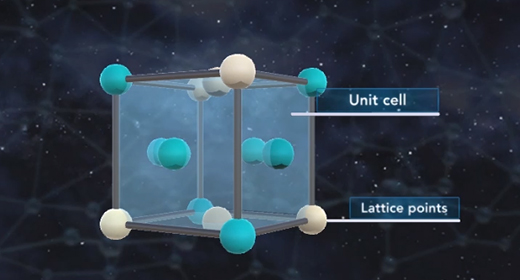
Upon completion of this module, you should be able to know what distinguishes amorphous solids from crystalline solids and describe the general structure of amorphous solids. The particles that constitute amorphous solids are not organized in a consistent pattern, which differentiates them from crystalline solids. Crystalline solids are highly ordered. They are comprised of repeating units of particles arranged in a fixed pattern. Due to their patterned structure, crystalline solids often have flat surfaces and definite shapes, while the contours and shapes of amorphous solids tend to be highly irregular.

The topic "Amorphous Solid" is available as part of this package. Subscribe this package to get access to this topic.