Activation Energy and Temperature Dependence of Rate Constants
Topic Description
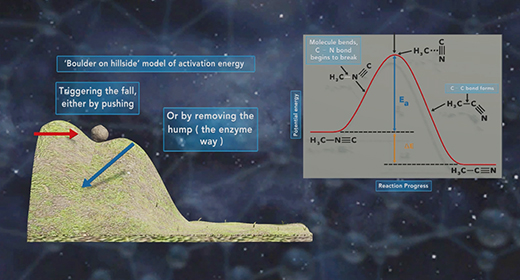
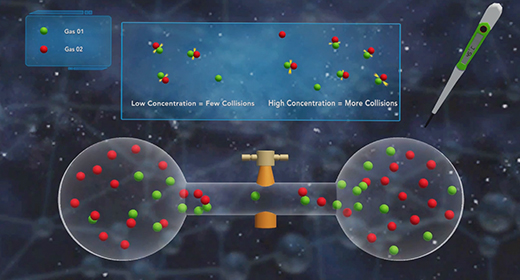
Upon completion of this module, you should be able to define activation energy, explain the difference between effective and ineffective particle collisions, and use the Arrhenius equation to calculate a rate constant. The activation energy (Ea) is the minimum amount of energy needed to initiate a chemical reaction. In a chemical reaction, reactant particles need to collide with enough activation energy to form the products. Reactions are typically carried at a particular temperature. At a particular temperature reactant particles have average kinetic energy.

The topic "Activation Energy and Temperature Dependence of Rate Constants" is available as part of this package. Subscribe this package to get access to this topic.