Acids and Salts
Topic Description
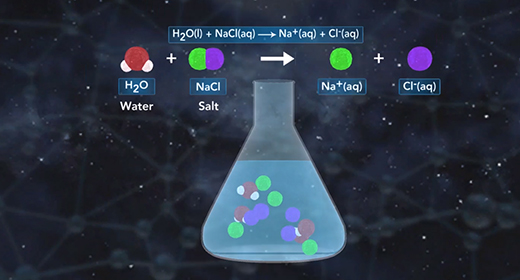
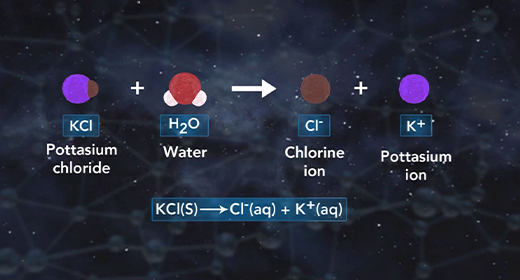
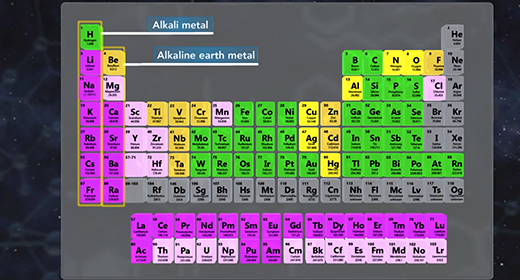
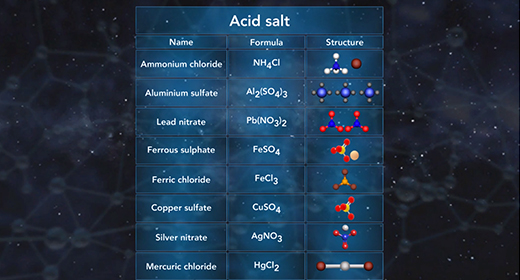
Upon completion of this module, you should be able to predict if a salt solution will be basic, acidic, or neutral and write the chemical reactions used to predict if a salt solution will be basic, acidic, or neutral. Salts are ionic compounds. When a soluble salt dissolves in water, it dissociates into its ions. Salt hydrolysis happens when one or both of the ions of a salt react with water forming either an acidic or basic solution. This is called a hydrolysis reaction.

The topic "Acids and Salts" is available as part of this package. Subscribe this package to get access to this topic.