Acid Base Properties of Water
Topic Description
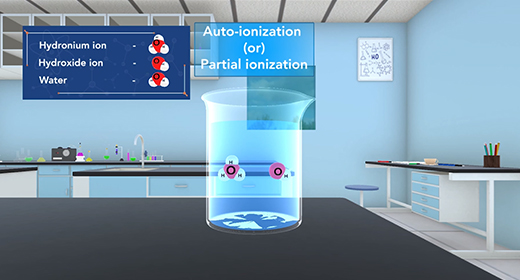
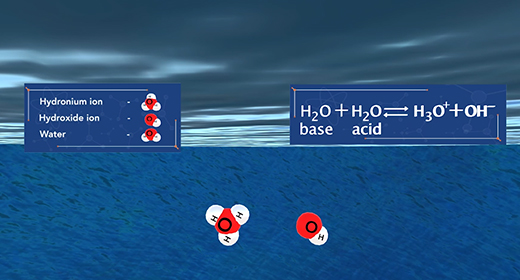
Upon completion of this module, you should be able to understand the acid-base properties using the Bronsted-Lowery theory and explain the autoionization of water. Bronsted-Lowry defined an acid as a proton donor and a base as a proton acceptor whether a substance is an acid or base depends on how the substance behaves in a given situation. In other words, for two different reactions, the same substance, such as water, can act as an acid in one reaction (donating a proton) while serving as a base in another reaction (accepting a proton).

The topic "Acid Base Properties of Water" is available as part of this package. Subscribe this package to get access to this topic.