The Bohr's Theory of Hydrogen Atom
Topic Description
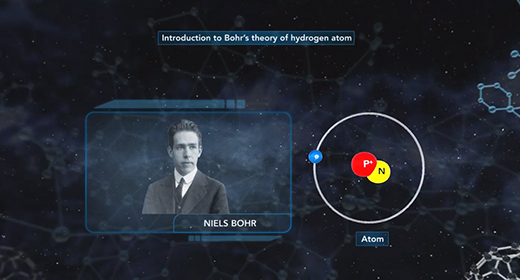
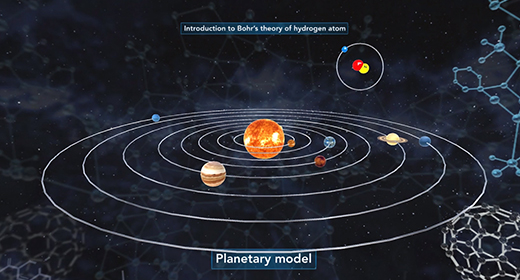
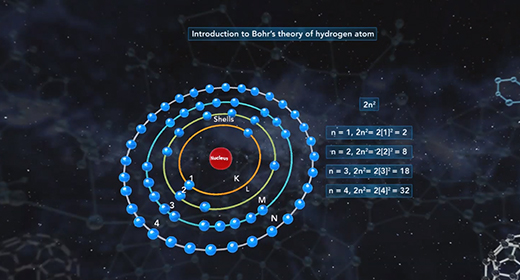
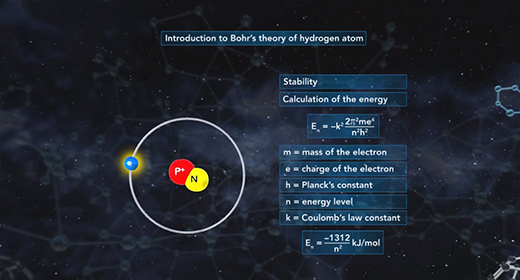
Upon completion of this module, you should be able to understand Bohr’s model of the atom and know the advantages of Bohr’s model of the hydrogen atom. In 1913 Niels Bohr proposed a new model of the atom. Bohr imagined hydrogen’s electron circling the nucleus in quantized energy levels that fit hydrogen’s emission spectrum. Bohr’s model for a hydrogen atom is often called the planetary model because Bohr believed that hydrogen’s electron orbited the nucleus in certain selected orbits in much the same way that planets orbit the sun.

The topic "The Bohr's Theory of Hydrogen Atom" is available as part of this package. Subscribe this package to get access to this topic.