Dalton's Law of Partial Pressure
Topic Description
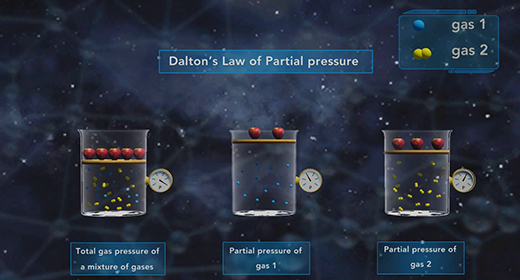
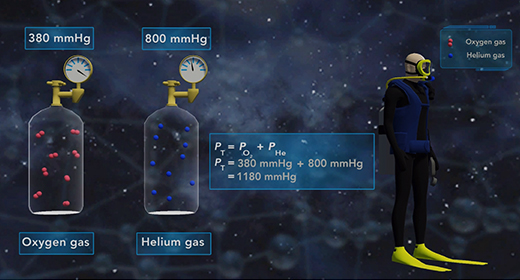
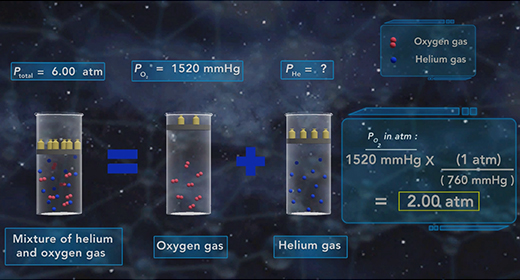
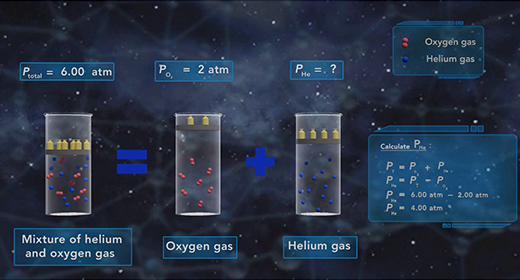
Upon completion of this module, you should be able to understand Dalton’s Law of Partial pressure and solve problems based on Dalton’s law of Partial Pressure. In 1803, John Dalton (1766 – 1844) proposed a gas law which was popularly known as Dalton’s Law of Partial pressure. This law states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas in that mixture.

The topic "Dalton's Law of Partial Pressure" is available as part of this package. Subscribe this package to get access to this topic.